Background: Autologous stem cell transplantation (ASCT) after induction treatment is standard of care for multiple myeloma (MM) for the last 30 years. Two transplantations up-front (so-called tandem transplant) were standard of care for all MM patients. With the use of new antimyeloma agents (such as proteasome inhibitors, immunomodulators and especially monoclonal antibodies) this approach has changed and tandem transplant is now reserved mainly for patients with high-risk disease, defined by revised international staging system (R-ISS). There have been reports which cast further doubts on the value of the more aggressive tandem ASCT approach, as well as reports suggesting higher incidence of secondary primary malignancies (SPM) after high dose therapy and ASCT.
Aims: To describe long-term outcomes of MM patients treated with ASCT (single, delayed, and tandem) and the rate of secondary primary malignancies.
Methods: a retrospective analysis of outcomes of 577 MM patients who underwent 865 ASCT procedures between 1993 and 2017 was performed. Data were collected from 3 transplant centres and 9 other haematological centres in which MM patients were treated pre- and post-transplant. During that period treatment strategies changed significantly from single to tandem to salvage second ASCT.
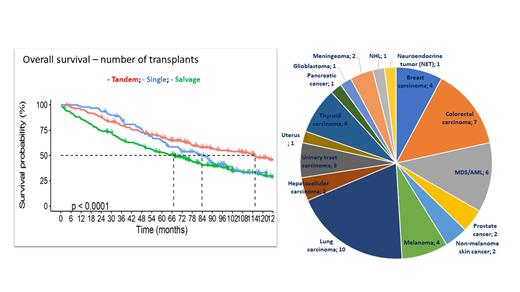
Results: there were 577 patients included. Median age at first transplant was 58 years (range 22 - 74). There were 295 female and 282 male patients. 346 patients received VAD (vincristine, doxorubicin, dexamethasone) and 231 received bortezomib and/or thalidomide (PI/IMiD) based regiments as induction therapy. All patients received high dose melphalan for pretransplant conditioning, 32 in combination with total body irradiation. Tandem ASCT (defined as second transplantation performed within 6 months after the first) was performed in 226 patients, single ASCT in 262 and salvage second (after relapse and/or progression) in 89 patients. Median overall survival (OS) for the entire group is 81.9 months and median progression free survival (PFS) 38.9 months. Tandem ASCT in comparison to single and second salvage transplantation resulted in statistically significant better OS (115 vs. 83 vs. 67 months respectively, p < 0.0001) and PFS (48 vs. 32. vs. 28 months respectively, p < 0.0001). There was no difference in outcomes regarding type of induction therapy, VAD vs. PI/IMiD based: median OS was 83 vs. 80 months, respectively (p=0.39) and median PFS was 39 vs. 38 months, respectively (p=0.15). 17.6% percent of patients who were treated with tandem ASCT are progression-free more than 10 years after the procedure while 10-year OS is 31.9%. 51 patients (8.8%) developed secondary malignancies (SPM). Most common SPM were lung carcinoma (10 patients), colorectal carcinoma (7 patients), myelodysplastic syndrome/acute myeloid leukaemia (6 patients), breast carcinoma (4 patients) and melanoma (4 patients). Of those 19 died due to SPM (3% of the entire patient group). There was no correlation between number of ASCT and SPM (p=0.87).
Conclusion: in our group of patients tandem ASCT resulted in superior OS and PFS in comparison to single and/or salvage ASCT. Interestingly, we did not observe benefit of new drugs (PI/IMiD) in induction therapy regarding outcomes. This peculiar result might be due to rather late availability of these drugs in front line settings (bortezomib and thalidomide since 2015) and time period taken for analysis (up to 2018). SPM rate was similar to other reports and did not show significant correlation with number of ASCT. More than 15% of patients treated with tandem ASCT experienced very prolonged PFS and almost 32% had OS longer than 10 years.
Disclosures
Batinic:Janssen: Honoraria; Pfizer: Honoraria; Amgen: Honoraria. Dreta:Takeda: Honoraria; Roche: Honoraria. Radić-Krišto:Janssen: Honoraria. Sinčić Petričević:Janssen: Honoraria; Amgen: Honoraria. Krecak:Janssen: Honoraria. Piršić:Amgen: Honoraria. Jakšić:Janssen: Honoraria. Rinčić:Takeda: Honoraria; Janssen: Honoraria. Valković:Janssen: Honoraria. Bašić-Kinda:Roche: Honoraria; Amgen: Honoraria; Janssen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal